Our Products
Our technological efforts have been focussed on developing innovative, superior, affordable, diagnostic catheter products for the following medical specialty and its important aspects:
- Cardiac Electrophysiology
- Cardiac Conduction
- Cardiac Arrhythmia
- Electrophysiology Diagnostic Catheters
Cardiac Electrophysiology
This sub-specialty is a study of mechanisms, functions, and performance of electrical activities of the heart. Impulse initiation and conduction, resting membrane potential, action potential, and refractory periods are the major, related aspects of cardiac electrophysiology.
The cardiac tissues have selectively-permeable cell membranes, each of which is composed of a lipid bi-layer and contains specialized proteins that form channels with gates that allow passage of certain ions at specific times between the intra- and extracellular regions. A cell membrane's electrical potential gradient is a result of positive-ions flow which may occur directly to and from the extracellular region through the channels in the membrane, or between adjoining cardiac cells through gap junctions, which are critical for the normal rapid propagation of electrical activity throughout the heart. The cell membranes are at rest when their intracellular region is electrically negative as compared with their extracellular region. Also, normally, at the resting state the intracellular region has more negative than positive ions.
During each cardiac cycle, the channels open and conduct ionic current for only a very short period which is governed by their physiologic activation and inactivation gates, both of which must be open in order for current to flow. At rest, nearly all of the activation gates are closed; and although inactivation gates are open.
Also, the electrical potential of the resting cell membrane is normally maintained at a certain range of negative millivoltage and is aided by sodium and potassium ions in the cell membrane fluid. The cardiac cell membranes maintain a high intracellular to extracellular potassium- concentration and a low intracellular to extracellular sodium- concentration. Also, a cardiac cell of the membrane at rest is permeable to potassium ions and relatively impermeable to sodium, calcium, and chloride ions. Therefore, the cell membrane at rest produces a negative electrical potential after passive diffusion of potassium ions out of the cell and due to the inability of sodium ions to enter the cell or due to lack of influx of sodium ions.
Normally, an electrical stimulus beginning in contiguous cells causes depolarization of the cell membrane, which is neutralization of the resting negative potential by influx of the positive ions of sodium and calcium and results in generation of an action potential in nearby cells. The action potential is a voltage change over time during the depolarization/repolarization of the cardiac cells. In order for a cell to depolarize, it, for various cardiac tissues, must reach a threshold potential at which sodium influx increases and potassium efflux decreases. The influx of negative ions of chloride and outflow of the potassium ions, on the other hand, causes repolarization or even hyperpolarization of the cell membrane. The net current shifts determine the actual membrane potential. There are 5 phases of a ventricular action potential, which are as follows:
- Phase 0: upstroke with rapid sodium ion influx–to depolarize
- Phase 1: rapid repolarization with rapid influx of chloride ion and an efflux of potassium ion
- Phase 2: plateau that represents a concurrent influx of calcium ion chloride ion
- Phase 3: final repolarization with outflow of potassium and decreased influx of calcium
- Phase 4: depolarization of the cells.
A cardiac cell is excitable when it has a capability to respond to an external stimulus by depolarizing and forming an action potential. Also, a cardiac cell membrane's voltage potential depends upon the opening and closing states of the activation and inactivation gates of the cell membrane's channels. When an external stimulus, originated from an electrode or from an adjacent cardiac cell, arrives at the nearby cells, the membrane potential reaches a certain voltage and activation gates open quickly allowing influx of sodium ions. After a few milliseconds, the inactivation gates close and prevent further sodium ion influx. Once the inactivation gates close, the cell cannot reopen the channel to generate another action potential no matter how strong a stimulus is applied. At the time, the cardiac cell is inexcitable or refractory.
The activation and inactivation gates gradually recover their ability to respond during phases 2 and 3 of the action potential. A stimulus applied near the end of phase 3 can open only those channels whose inactivation gates have returned to the open state.
A cardiac cell is in the absolute refractory period if a stimulus is applied near the end of phase 3 and when not enough inactivation gates are open to allow adequately enough sodium ion influx in order for this process to result in a self-sustaining action potential. A cardiac cell is in the relative refractory period if a stimulus is applied few milliseconds later and when more inactivation gates are open to conduct some but not a normal amount of ionic current, which, in this case, results in a slower upstroke in phase 0. A cardiac cell is fully excitable to result in a normal action potential if a stimulus occurs still later and encounter essentially all gates at the ready states.
A cardiac cell is in an effective refractory period during which transient depolarization may occur but no action potential may be propagated.
Cardiac Conduction
To significantly accomplish its hemodynamic function, the heart must be able to perform a coordinated contraction of myocardial cells approximately simultaneously while having its overall electrical activation occur very rapidly. Factors which aid in speed of cardiac cell-to-cell current transmission (electrical conduction velocity) are as follows: rapid development of the potential difference between cells, the magnitude of the excitatory current, and the resistance to intercellular current flow, which, in a large part, is mediated by gap junctions. Factors which may adversely hinder the electrical conduction velocity are the following: a decrease in the excitatory stimulus strength, decreased membrane receptiveness, stimulus occurring during relative refractory period, and increased resistance to axial current flow which, in other words, is decrease in number of gap junctions.
In the heart, the sinoatrial (SA) and atrioventricular (AV) nodes and the His-Purkinje system have specialized tissues, which act like both muscle and nervous tissues and are capable of initiating an electrical activity. Also, the cells of the specialized tissues have a characteristic of automaticity, which means an occurrence of spontaneous, repeated cellular depolarization that results in an action potential. Due to automaticity, the specialized tissue cells will spontaneously and repeatedly generate an electrical impulse during phase 4 of the action potential toward the threshold potential. The cardiac cells in other areas do not normally display automaticity. It can be stated that the specialized tissues are responsible for the conduction of electrical activity to the atria and ventricles of the heart.
Normally, an electrical impulse originates from the SA node, which is also known as a natural cardiac pacemaker and has the fastest intrinsic automaticity (firing) rate that determines the heart rate. The electrical impulse stimulates the cardiac cells and causes cardiac depolarization by reversing the polarity of the cardiac cells and making the inside of the cell become more positive relative to the outside of the cell, which reflects the electrical current flow to all cells along the conduction pathways. Cardiac repolarization occurs when the cells return to their original resting state.
In addition to the SA node, the AV node and His-Purkinje system, also have ability to function as redundant pacemakers whose automaticity rate is slower than that of the SA node. Cardiac depolarization from an impulse of the SA node, which is a dominant pacemaker, suppresses these redundant pacemakers. If the SA node fails to maintain its pacemaking activity, the AV node can allow a continuous cardiac electrical activity. The His-Purkinje system serves as a back-up subsidiary pacemaker in case the SA node or AV conduction fails. The electrical activity of the redundant pacemakers is normally acknowledged only when SA node rates fall below those of the redundant pacemakers whose emergence to sustain a heart rate when the dominant pacemaker fails is called an escape mechanism, which provides a slower heart rate.
On the surface EKG, the SA node automaticity is not recorded. The SA node impulse activates the internodal tracts and depolarizes the atrial myocardium, which results in atrial contraction and produces. the P wave on the surface EKG, indicating the depolarization of the atria. The beginning part of the P wave represents right atrial activation, whereas the ending part of the P wave represents activation of the left atrium with some overlap in the middle. The number of SA nodal impulses reaching the ventricles is controlled by the AV node.
The SA nodal impulse then depolarizes the AV node, the His bundle, the bundle branches, the Purkinje fibers and the ventricular myocardium.
Also, on the surface EKG, propagation of the impulse through the AV node and His bundle branch-Purkinje system is not recorded and occurs during the iso-electric PR segment on the EKG, which indicates the AV nodal and His-bundle conduction time. Ventricular myocardium depolarization aids in the conduction through the bundle branches and Purkinje fibers, which results in contraction of the ventricles and produces the QRS complex on the surface EKG.
Atrial repolarization follows atrial depolarization but is generally not discernible on the surface EKG. The recovery of the ventricular myocardium as a result of ventricular repolarization or re-attainment of resting membrane potential is represented with a T wave on the surface EKG, which follows the QRS complex.
It is noteworthy to mention that electrical depolarization of the atrial and ventricular myocardia is not similar to or concurrent with atrial and ventricular contraction. The electrical depolarization of these myocardia must precede the corresponding mechanical myocardial contraction; however, mechanical contraction occasionally may not follow the electrical depolarization or repolarization, for example, during electrical-mechanical dissociation.
Cardiac conduction, in summary, is as follows: an electrical impulse propagates through a specialized conducting system, which includes sinoatrial (SA) node; anterior, middle and posterior internodal tracts; atrioventricular (AV) node; His bundle; right and left bundle branches; anterior-superior and posterior-inferior divisions of the left bundle and the Purkinje fibers.
Cardiac Arrhythmia
Normally, Sinus rhythm of a heart occurs when the SA node, the natural cardiac pacemaker, spontaneously and repeatedly generates an electrical impulse which, in a waveform, propagates through the cardiac atria and ventricles and depolarize the myocardial structure by following the natural, specialized conduction pathways in the heart. The parasympathetic system normally slows the automaticity rate of the SA node from 100 beats per minutes to about 70 beats per minutes (bpm). The limits (the normal range) of the rate of the rhythm are 50bpm and 120bpm.
A heart rate that is faster than the normal range is termed tachycardia while a heart rate that is slower than the normal range is termed bradycardia. Also, disturbances in the rhythm are termed sinus arrhythmia.
Bradycardia
The normal sinus rhythm slows when there is a greater increase in the parasympathetic tone and a decrease in sympathetic stimulation, while the SA node is still pacing beats of normal conduction, the rhythm is termed sinus bradycardia. The threshold of the heart rate varies but is normally considered to be 50bpm.
Tachycardia
The heart rate is raised by reduction of parasympathetic tone or an increase in sympathetic stimulation. When conduction is normal and the rate is greater than 120bpm, then the rhythm is called sinus tachycardia.
Sinus Arrhythmia
Variations in heart rate from beat to beat are greater than would be expected from normal rhythm variation. Its irregularity is caused by fluctuations of autonomic tone that result in phasic changes of the automaticity (discharge) rate.
Blocks
During depolarization from the SA node to the ventricular myocardium, an interference with the conduction process is called heart block. There are three basic forms of heart block.
First Degree Atrioventricular Block
This occurs when there is a delay in conduction at the junctional area. This is not really a block, since conduction passes down the normal pathways, but it is referred to as first-degree atrioventricular block.
Second Degree Atrioventricular Block
When conduction fails to pass through the atrioventricular node or the bundle of His and when this occurs intermittently, it is known as second-degree atrioventricular block.
Third Degree Atrioventricular Block
This block occurs when atrial contraction is normal but there is no conduction to the ventricles. In this case, the atrial rhythm is independent of the ventricular rhythm, unless there is a presence of an accessory pathway that conducts antegrade to the ventricle.
Bundle Branch Block
It occurs when there is abnormal conduction through either the right or left bundle branches which causes a delay in the depolarization of part of the ventricular myocardium. The extra time taken for depolarization because of a presence of a slow pathway causes widening of the QRS complex on the surface EKG.
Delays
These are caused by degeneration within the SA node and characterized by long time-intervals between consecutive events of atrial depolarization. The causes and types of delays considered are as follows:
Dropped Beat
This delay is defined as a dropped beat when the time duration is a multiple of the basic sinus interval. An impulse will have formed within the SA node, but failed to reach the atria, which is also called sinoatrial exit block.
Sinus Pause
It is a delay of activation within the atria for a period to which the previous sinus interval is not a harmonic.
Sinus Arrest
It is a delay of activation within the atria for a longer period (than sinus pause) to which the previous sinus interval is not a harmonic. The patient is likely to experience syncope from the sinus arrest.
Escapes
In various parts of the heart, the backup, subsidiary (redundant) natural pacemakers or impulse originating foci develop slow and protective rhythms to take over the control of the heart. These rhythms are called escape rhythms because they occur when secondary sites for initiating depolarization escape from the normal suppression of the more active SA node that is the dominant natural pacemaker and has the fastest automaticity rate.
Atrial Escape
It occurs when the SA node slows down and a focus in the atrium takes over control of the heart. The P wave will be abnormal if the focus is away from the SA node. It will have a normal ventricular depolarization and repolarization cycle giving sinus morphology to the QRS complex and T wave.
Junctional Escape (Also nodal escape)
It occurs when the region around the atrioventricular node takes over to develop rhythm of the heart. Due to an absence of atrial contraction, the beat after the delay will show no P wave or one of a late, inverted morphology. Or the P wave is partially lost within the QRS complex. The morphology of the P wave depends upon how far away the initial focus lies in the region. The further away the focus, the later the atrial contraction. The wave will always be inverted because it traverses the atria in the opposite direction as compared with the sinus rhythm.
Ventricular Escape
It occurs when the SA node slows down and a focus in the ventricles takes over control of the heart. This results in an abnormal, wide QRS complex of a ventricular beat ending a sinus pause and then restoring the heart to sinus rhythm.
Premature Beats
These are called ectopic beats that occur when a focus causes an early or a premature contraction of a myocardium. When the focus is in the atria, atrial ectopic beats occur with normal ventricular operation, and when the focus is in the ventricular myocardium, broad QRS complex beats occur. More complex situations also produce aberrant and fusion beats.
Premature Atrial Contractions
It is also called an atrial ectopic beat, The beat is caused by the premature contraction of a muscle within the atrial myocardium. Two ectopic beats can appear as double beats if the SA node activates the atrial myocardium. After it has repolarized, the atria can perform a second contraction. Three or more premature beats are caused by a reentrant mechanism. Other ectopic activities include bigeminy--an ectopic beat every other contraction and trigeminy--an ectopic beat every third contraction.
Premature Ventricular Contractions
It is also called a ventricular ectopic beat, a ventricular premature complex or a ventricular extrasystole that occurs when a focus within the ventricles prematurely causes myocardial depolarization. The beat will have a wide QRS complex with either initially a high-energy negative, or positive, deflection whose direction depends upon the location of the focus. Two, three and more consecutive ventricular ectopic beats may be caused by a re-entrant mechanism. If there are more than one focus exists (multi-focal) then the QRS complex will exhibit more than one morphology at different times. As in the case of atrial ectopics, other ectopic activities include bigeminy--an ectopic beat every other contraction and trigeminy--an ectopic beat every third contraction.
Fusion Beats
It happens when the ventricular focus and contraction occur simultaneously as a conventional AV-node-driven contraction. In this case, the beat has a P wave but a broad QRS complex and inverted T wave.Supraventricular Arrhythmias
These arrhythmias are caused by abnormal conduction and have origins within the atria. Also, they have conduction irregularities that bypass the AV node using non-standard pathways. The most common of these arrhythmias are as follows:
Supraventricular Tachycardia (SVT)
It is also called atrial tachycardia and occurs when the electrical impulse does not originate from the SA node. The patient may feel dizzy, attacks can last for a few beats or for many hours.
Atrial Flutter
Often, a premature beat, possibly supported with a reentry mechanism or a single ectopic focus, initiates the atrial flutter, creating a rhythm of 240-350 bpm. Due to the refractory nature of the conduction pathway at the higher rate and a resulting variable block within the junctional area, some of atrial contractions (impulses) are not transmitted to the ventricles. The atrial waves exhibit saw-tooth morphology.
Atrial Fibrillation
This arrhythmia is often caused by reentrant excitation within the atria with multiple, emergent foci and reentry circuits. This atrial arrhythmia has a rate of over 350 bpm , which results in a loss of normal atrial contraction due to the ineffective trembling of the atrial myocardium.
Wolf-Parkinson-White Syndrome (WPW)
In this syndrome, the conduction propagates through an abnormal pathway and bypasses the AV node and results in early activation of the ventricles from the bypass tract. Also, in this syndrome occurs two activation sequences, one from the bypass tract and the other from the AV node. The EKG changes associated with WPW syndrome include: shortened PR interval, a delta wave and a broad QRS interval.
Ventricular Arrhythmias
These arrhythmias are caused by the depolarization wave that propagates through the ventricles via irregular and abnormal pathways. Repolarization also occurs through different pathways. Above 120bpm, this rhythm is called Ventricular Tachycardia. The most common of these arrhythmias are as follows:
Accelerated Idioventricular Rhythm
This is also called accelerated ventricular rhythm.
Ventricular Tachycardia
This is often initiated by premature ventricular complexes and continued by re-entry mechanisms. In some cases, this arrhythmia can degenerate into ventricular fibrillation if the heart rate increases dramatically.
Torsades de Pointes
This is also called polymorphic ventricular tachycardia, which is due to a movement in the reentrant mechanism
Ventricular Fibrillation
This arrhythmia is a form of cardiac arrest and occurs when ventricular contractions are rapid, irregular and ineffective due to emergence of multiple foci.
Asystole
This is also called ventricular standstill and exhibits cessation of ventricular activity.
Electrophysiology Diagnostic Catheters
The cardiac Electrophysiology (EP) study is crucial for diagnosing and treating cardiac arrhythmia, which has been gradually increasing over the years due to the fact that the people are living longer. The EP physicians who practice medicine in full service hospitals or cardiology institutes in the USA are motivated to buy technologically superior yet affordable products with novel features. Also, the EP physicians often prescribe specialty or custom EP catheters for their certain arrhythmia patients who may be refractory to clinical procedures performed with conventional EP catheters.
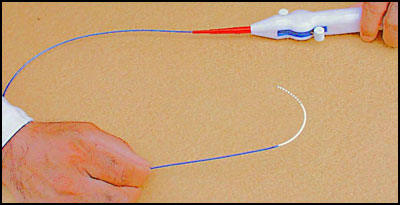
Moreover, due to their budget constraint and/or cost consciousness, the EP physicians and the hospital buying groups are always searching for affordable and superior solutions to the existing EP catheters/systems. So, the physicians often rely on substitute and complementary catheter products to carefully and skillfully perform the clinical procedures, which often lead to device-use difficulties/failures, patient trauma/injuries and unnecessarily increased periprocedural time, exposing the patients to further potential hazards (e.g., prolonged radiation exposure).
Therefore, recognizing their needs, we have developed state-of-the-art cardiac electrophysiology catheter products, which will provide EP physicians with advanced and advantageous features, superior performance, and price affordability, not offered in the competitive products.
We offer our innovative electrophysiology diagnostic catheters with the following features and characteristics in 3, 4, 5 and 6 French sizes by the trade names of SlenderCath™, Apica® I, Apica® II and Aura®, respectively:
Advantageous Features
- Bi-directional Steerability for Easier Vessel Access, Better Catheter Maneuverability and Consistent Distal Tip Placement/Replacement
- Unidirectional Steerability Option Available at A Lower Price
- Fixed-curve Catheters Encompass all Conventional and Custom Curves
- Ergonomic Catheter Handle
- Intuitive Steering Mechanism
- Low Steering Actuation Force
- Built-in Deflectable Distal Curve Lock
- Gradual Stiffness Transition between the Distal and Proximal Shafts
- Small to Large Deflectable Distal Shaft Curves
- Smooth Curve Shape of the deflectable Distal Shaft
- High-degree Curve Angle of the Deflectable Distal Shaft
- Up to 10 Electrodes for Small French-size Catheters (3Fr, 4Fr and 5Fr)
- Up to 20 Electrodes for Slightly Larger French-size Catheters (6Fr)
Superior Performance
- Ease of Catheter Advancement to Access A Targeted Endocardium Site
- Tortuous Anatomical/Vascular Path Trackability
- High Torque Transmission to the Distal Shaft/Tip
- Reliable Steerability
- Consistent Curve Maintainability of the Deflectable Distal Shaft
- Optimal Electrical Performance
Price Affordability
- 30% Lower than the Competitive Catheter's Sales Price
- The Same Price for the Different French Size Catheters with the Same Number of Electrodes and the Same Distal Curve and Catheter Length
